FEBRUARY IS AMERICAN HEART MONTH! HOW IS HEART HEALTH AFFECTED IN PREGNANCY AND POSTPARTUM?
In this blog, I’ll be reviewing the incidence of cardiovascular disease (CVD) in pregnancy, and postpartum. Additionally, I’ll present a case presentation in an effort for the reader to reflect on the learned knowledge from the blog post in the context of the presented case. I’ll also address the challenges associated with diagnosing CVD in pregnancy, and postpartum, while highlighting the signs, and symptoms as well as risk factors for CVD. In closing, I’ll conclude with key takeaways.
Incidence: Cardiovascular disease (CVD) is one of the leading causes of maternal mortality in the United States accounting for >33% of all pregnancy-related deaths in the U.S. One of every three intensive care admissions in pregnancy, and the postpartum period are related to CVD. CVD is under-recognized in pregnant, and postpartum women with rates higher among African-American women.
It’s estimated that 25% of deaths caused by cardiovascular disease in pregnancy or during the postpartum period may have been prevented if CVD had been diagnosed earlier. Only a small fraction of women who die from CVD have a known diagnosis of CVD prior to death. The majority of women who die from CVD present with symptoms either during pregnancy or after childbirth

CMQCC, 2017. CARDIOVASCULAR DISEASE IN PREGNANCY AND POSTPARTUM TOOLKIT
Diagnostic Challenges and Signs/Symptoms: Signs, and symptoms of normal pregnancy, and postpartum mirror CVD making it difficult to diagnose. This is due to the normal physiological changes that occur in pregnancy, and the postpartum period. However, a diagnosis of CVD should be suspected when symptoms are severe (see red flags below) with vital sign abnormalities, and underlying risk factors. Having an increased awareness of the prevalence of CVD, and a high index of suspicion, along with preconception counseling, and referral to a higher level of care can prevent adverse maternal outcomes.
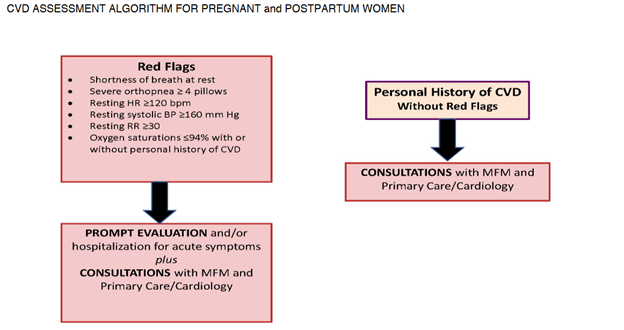
CMQCC, 2017. CARDIOVASCULAR DISEASE IN PREGNANCY AND POSTPARTUM TOOLKIT
Risk Factors: Risk factors for the development of CVD in pregnancy, and postpartum include polycystic ovary syndrome, infertility, adverse pregnancy outcomes such as hypertensive disorders of pregnancy, gestational diabetes, preterm delivery, and intrauterine growth restriction.
Key Takeaways:
- Symptoms related to the normal physiological changes of pregnancy should improve in the postpartum period.
- The highest risk period for CVD worsening is between 24-28 weeks of pregnancy or postpartum.
- Emergency Room visits for dyspnea (shortness of breath) should heighten suspicion level for CVD.
- Postpartum dyspnea or a new onset cough should heighten suspicion for CVD.
- New onset asthma is rare in adults.
- Bilateral crackles are likely related to congestive heart failure (CHF).
- Bilateral infiltrates on chest x-ray may be due to heart failure rather than pneumonia.
- Hypertension and diabetes in pregnancy increases the risk of CVD.
- Healthy lifestyle changes can reduce future CVD risk by 4-13%.

References:
ACOG, 2019. Pregnancy and heart disease.
AHA, 2020. Cardiac arrest in pregnancy in-hospital ACLS algorithm.
AWHONN, 2023. Obstetric patient safety ob emergencies workshop, 3rd ed.
CMQCC, 2017. Cardiovascular disease in pregnancy and postpartum toolkit.
P.S. COMMENT AND SHARE: What is your experience with cardiovascular disease in pregnancy or in the postpartum period? Have you been involved in an adverse outcome as a result of a CVD diagnosis, or failed diagnosis?
