THE LINK BETWEEN UTERINE ACTIVITY IN LABOR, FETAL (IN)TOLERANCE, AND LIABILITY
In this blog, I’ll review basic uteroplacental physiology in labor, as well as the components of a complete uterine activity assessment. I’ll highlight some litigation trends related to uterine activity in labor, and close with risk management approaches.
Physiology Basics: The uterus is a muscle that contracts in labor. Blood supply to the uterus, placenta, and fetus is dependent on maternal blood pressure, as well as uterine activity (e.g. contractions). With every labor contraction, the blood supply to the uterus, placenta, and fetus is reduced by 60%.
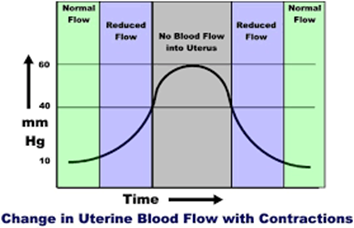
- 75% of fetal hypoxia cases in the intrapartum period occur slowly, and progressively from the impact of uterine contractions over a period of time, rather than from an acute event.
- Labor contractions can reduce the fetal partial pressure of oxygen (PaO2) by 25%. The healthy fetus is able to tolerate this, however, a high-risk fetus (e.g. fetus sustaining recurrent significant deceleration’s, fetal growth restriction, maternal history of chronic hypertension), may not be able to tolerate this reduction.
- The relaxation time between contractions results in the ability for restoration of maternal-fetal gas exchange, and allows the fetus to return the PaO2 to normal levels, and clear carbon dioxide (CO2), and lactate.
- Fetal lactate is a by-product of fetal anaerobic metabolism related to periods of hypoxia (low oxygen levels in the fetus’ body tissues.)
Fetal (In)Tolerance to Labor Contractions: Excessive uterine activity can have an adverse effect on fetal oxygenation, and the acid-base status of the fetus. Excessive uterine activity can result in decreased fetal cerebral oxygen saturation, as well as fetal acidemia (fetal blood with high levels of acid, or a low pH). Excessive uterine activity can result in birth injuries, specifically, hypoxic ischemic encephalopathy, subsequent cerebral palsy, and possible death.
Excessive uterine activity can have an adverse effect on uterine re-perfusion as well. In the presence of anaerobic metabolism, elevated uterine lactate levels can develop. This can result in inadequate labor contractions, ultimately leading to Pitocin augmentation, cesarean delivery, postpartum hemorrhage, and associated sequelae.
The number one cause of fetal intolerance to labor contractions is inadequate re-perfusion due to inadequate relaxation time between contractions.
- Uterine activity affects the fetal base deficit (BD)
- There is a minimal effect from labor onset to 4cm dilation
- From 4cm dilation to complete dilation, the mean BD increases by approximately 1mmol/L every 3 hours due to the increased frequency, and intensity of contractions
- In second stage labor, the BD will increase by 1mmol/L every 1 hour due to the increased strength, and frequency of contractions, as well as maternal pushing efforts
- The effects on BD are more significant with an interruption of the maternal fetal oxygen pathway (e.g. presence of variable, late, and/or prolonged decelerations), as well as with excessive uterine activity
- Umbilical artery pH is reduced, while lactate levels are increased with increasing uterine activity, intrauterine pressure, and resting tones
Complete Assessment of Uterine Activity: The assessment of the fetus should not be performed in the absence of a complete assessment of uterine activity. Components of uterine activity assessment, and documentation include –
- uterine contraction frequency: The number of contractions in a 10-minute period
- normal frequency ranges from 2 – 5 contractions per 10 minutes
- frequency alone is only a partial assessment of uterine activity
- identified as abnormal with:
- > 5 contractions in 10 minutes averaged over 30 minutes = uterine tachysystole
- tachysystole includes spontaneous, and induced contractions
- requires treatment even if the fetal heart rate (FHR) tracing is normal
- > 5 contractions in 10 minutes averaged over 30 minutes = uterine tachysystole
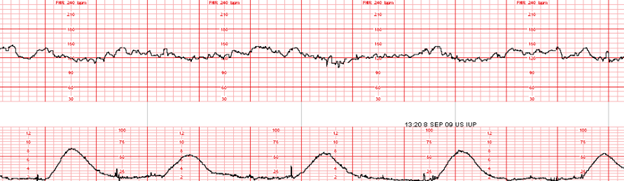
- duration of uterine contractions: The time from the onset of a contraction, to the offset of a contraction measured from baseline resting tone
- ranges from 45 – 80 seconds
- strength or intensity of uterine contractions: The peak of a contraction minus the resting tone
- 25 – 50 mmHg (millimeters of mercury) in the first stage of labor
- may rise to >80 mmHg in the second stage of labor
- contractions palpated as “mild” would peak at < 50 mmHg if measured internally. Contractions palpated as “moderate” or greater would peak at 50 mmHg or greater if measured internally
- resting tone of uterus: The intrauterine pressure when the uterus is not contracting
- the average resting tone during labor is 10 mmHg. If assessment if performed by palpation (touching), assessment should palpate as “soft”: easily indented
- increased resting tone = hypertonus
- resting tone > 20-25mmHg, or uterus does not palpate soft between contractions
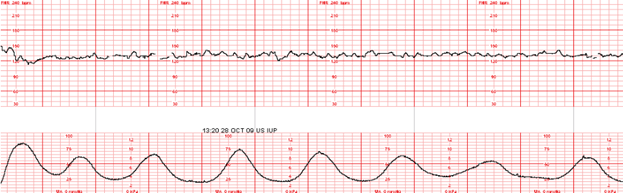
- relaxation time between uterine contractions: The time-frame from the end of one contraction to the beginning of the next
- this is usually 60 seconds or more in the first stage of labor, and 45 seconds or more in the second stage
- frequently confused with resting tone
- Montevideo units (MVUs): Assessed and documented if an intrauterine pressure catheter (IUPC) is in place. The average intensity of contractions in mmHg multiplied by the number of contractions in 10 minutes
- MVUs range from 100 – 250 MVUs in the first stage of labor. MVUs may rise to 300 – 400 MVUs in the second stage of labor
- “adequate” labor contractions are defined as > 200 MVUs
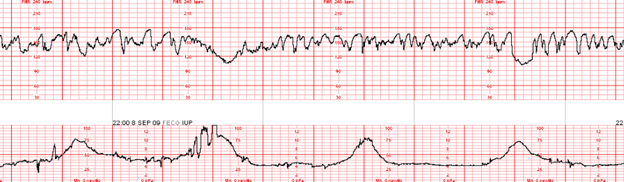
What is Excessive Uterine Activity? All definitions for excessive uterine activity apply to both spontaneous, and induced, or augmented labor. The evaluation of the presence, or absence of uterine tachysystole, in addition to evaluation of contraction duration, uterine resting tone, and relaxation time between contractions.
- Tachysystole: contraction frequency >5 in 10 minutes, averaged over 30 minutes.
- Hypertonus: uterine resting tone exceeding 20 -25 mmHg with an IUPC, or a uterus that doe not return to “soft” by palpation during relaxation between contractions.
- Inadequate relaxation time: less than 60 seconds of uterine relaxation between contractions during the first stage of labor; less than 45 seconds of uterine relaxation between contractions in the second stage of labor.
- Excessive contraction duration (i.e. prolonged contractions, tetanic contractions, uterine tetany.) A series of single contractions lasting 2 minutes or more.
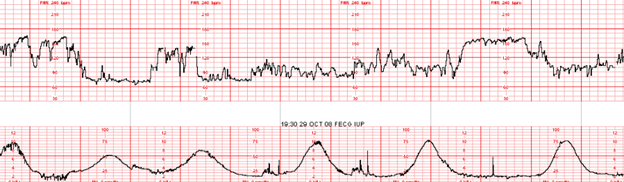
Management of Excessive Uterine Activity: Management of excessive uterine activity should not be based on the presence or absence of FHR changes.
The goal of management is to identify, and promote normal uterine activity, and correct the underlying cause of any type of excessive uterine activity.
- maternal position change to side-lying
- administration of an intravenous fluid bolus
- removal of cervical ripening agents, or decrease or discontinue the use of oxytocin
- use of a tocolytic (i.e., terbutaline) if the above interventions were ineffective, or the excessive uterine activity occurs in the presence of FHR changes indicative of interrupted fetal oxygenation
EXCESSIVE UTERINE ACTIVITY SHOULD TRIGGER INTERVENTIONS, REGARDLESS OF THE FHR STATUS
Uterine Tachysystole, and Excessive Uterine Activity: A Common Area of Liability
Case Study (10/07/2020)
A 28-year-old gravida 2, para 1 at 37 weeks gestation presented for a scheduled Pitocin induction of labor for preeclampsia without severe features.
Admission assessments were unremarkable, with a baseline favorable cervical examination of 3/80/-1. The labor course was complicated by a recurrent Category II FHR tracing in the presence of excessive uterine activity. The patient progressed to complete dilation, attempted to push with contractions, resulting in an onset of fetal bradycardia. An emergency cesarean delivery occurred resulting in delivery of a viable male weighing 7#2oz with Apgar scores of 1/3/3/3/5. A venous cord gas revealed metabolic acidemia: pH 6.94, BD 14.6. An arterial cord gas was unable to be obtained.
The newborn was diagnosed with hypoxic ischemic encephalopathy (HIE), and received therapeutic hypothermia. The newborn had an onset of seizures at seven hours of life. A brain MRI was performed on day of life five revealing a watershed pattern of injury. The newborn was discharged home with the parents on day of life 16. The infant suffers from spastic cerebral palsy with profound neurodevelopmental delays.
EFM Tracing with Annotated Segment of Ob Provider Note:
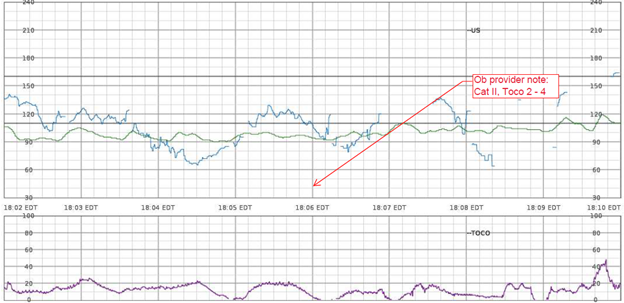
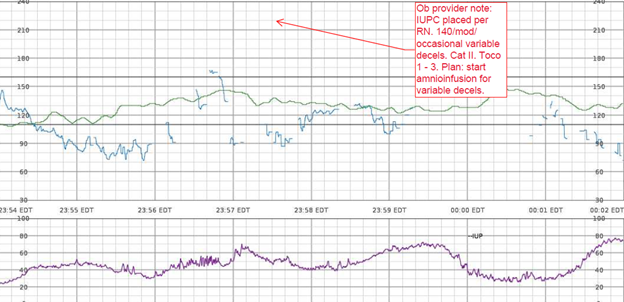
Nurses Notes: RN notes indicate a “normal” uterine contraction pattern, normal duration of contractions, and a “soft” resting tone throughout labor.
Q: What assessments are missing specific to uterine activity?
A: contraction duration, resting tone in mmHg, relaxation time between contractions, MVUs in the presence of an IUPC, uterine, and fetal responses to interventions
Litigation Case Theme: Failure of the perinatal team to treat a Category II FHR tracing in the presence of uterine tachysystole, and excessive uterine activity resulting in HIE, and subsequent permanent neurological injuries.
Plaintiff Allegations:
Failure to appropriately identify and treat uterine tachysystole, and excessive uterine activity in the presence of a Category II FHR tracing in a timely manner
Failure to discontinue oxytocin in the presence of excessive uterine activity, and a Category II FHR tracing
Inappropriate oxytocin management
Failure to initiate intrauterine resuscitation
Failure to follow oxytocin orders, and policy
Failure to activate the chain of command when there was clinical disagreement between the nurse, and responsible physician
Defenses:
The fetal injury likely occurred during the antenatal period; therefore, actions during labor and delivery had no impact on the outcome
The standard of care was adhered to
Documentation reflected prompt, and appropriate actions by the perinatal team
Electronic fetal monitoring cannot be used as a diagnostic tool; therefore, birth injury cannot be attributed solely to FHR interpretation
Standard of Care Takeaways (Risk Management Approaches): the evaluation of uterine activity must occur, and is equally as relevant as the assessment of the fetal heart rate.
- Hospital systems should have clear definitions of tachysystole, and excessive uterine activity as clinical management, and policies/procedures should guide expected interventions when tachysystole or excessive uterine activity is identified.
- Ensure organizational agreement on guidelines specific to uterine activity, not just management of tachysystole
- All members of the perinatal team (physicians, residents, midwives, nurse practitioners, nurses) should be aware of the clinical criteria established for uterine tachysystole, and excessive uterine activity.
- Most episodes of uterine tachysystole, and excessive uterine activity, occur as a result of the administration of oxytocin (60%).
- Treat uterine tachysystole, and excessive uterine activity by decreasing or discontinuing oxytocin, even in the presence of a Category I FHR tracing.
- Avoid prolonged periods of uterine tachysystole, and excessive uterine activity that leads to progressive deterioration of the fetal status, and subsequent indeterminate (Category II) or abnormal (Category III) FHR patterns.
- Treatment for uterine tachysystole, and excessive uterine activity should not be delayed until there is evidence of an indeterminate (Category II) or abnormal (Category III) fetal status.
- All members of the perinatal team should be consistent with their assessments, interventions, and communications with colleagues.
- Nurses should advocate for patient safety if they feel pressured to increase oxytocin rates during uterine tachysystole and/or indeterminate (Category II) or abnormal (Category III) FHR patterns.
- Remain current with your professional organization, and organizational literature
- Consistently communicate, and document utilizing the standardized nomenclature including all characteristics of uterine activity assessment (frequency, intensity, duration, resting tone, relaxation time).
- Ensure all members of the perinatal team are aware of the components of a complete assessment of uterine activity in labor.
- Recognize that contraction frequency alone is a partial assessment of uterine activity. Other components are equally important: duration, intensity, resting tone, relaxation time. Attain, and maintain adequate uterine activity.
- Be able to identify signs of interruption in the maternal fetal oxygen pathway.
Conclusion: The assessment of uterine activity during labor is crucial, and is considered a patient safety issue. Applying known parameters to the assessment of uterine activity influences management decisions, forms the basis of safe use of labor stimulants, and provides a means of defining excessive uterine activity among the multidisciplinary perinatal team.
The goal of this blog post is to improve birth outcomes, reduce liability risk to perinatal care providers, educate perinatal team members to avoid the risk of recurrence of errors, while supporting the reduction of obstetrical related medical malpractice lawsuits.
References:
AAP, ACOG (2017). Guidelines for Perinatal Care
ACOG (2009). Induction of Labor
ACOG (2010). Intrapartum Fetal Heart Rate Tracings
AWHONN (2014). Perinatal Nursing
AWHONN (2021). Perinatal Nursing
AWHONN (2022). Intermediate Fetal Monitoring Course
Miller et al., (2022). Mosby’s Pocket Guide to Fetal Monitoring
Rimsza et al., (2024). Association between Elevated Intrauterine Resting Tone during Labor and Neonatal Morbidity. American Journal of Perinatology
Ross et al., (2002). Use of umbilical artery base excess: algorithm for the timing of hypoxic injury. American Journal of Obstetrics & Gynecology
Turner et al., (2020). The physiology of intrapartum fetal compromise at term. American Journal of Obstetrics and Gynecology
COMMENT AND SHARE: What is your experience with a lawsuit involving uterine tachysystole, or excessive uterine activity? What was the case theme? Did the case go to trial? What was the case verdict?